Valence Bond Theory :
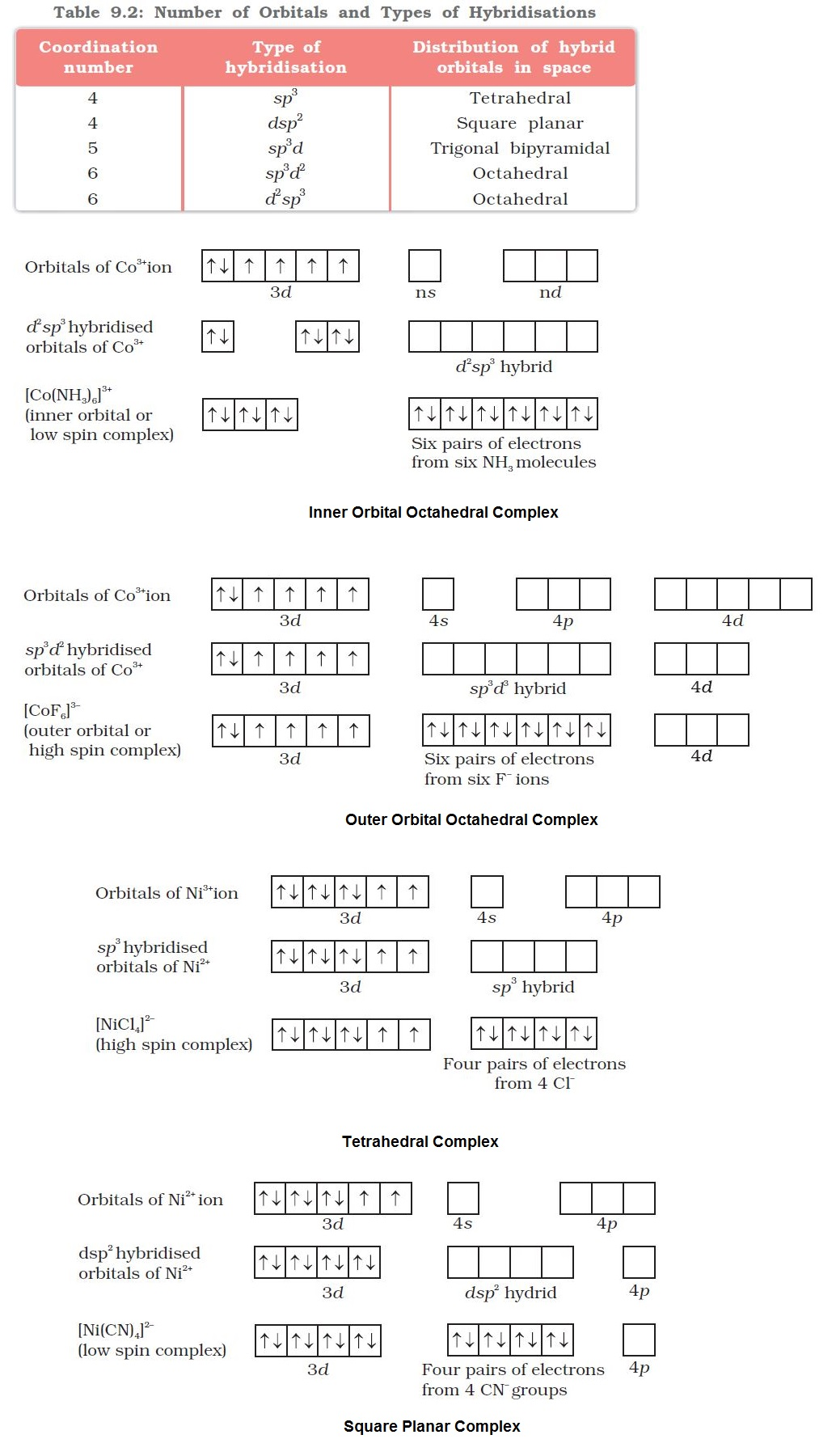
`=>` According to this theory, the metal atom or ion under the influence of ligands can use its `color{red}((n-1)d, ns, np)` or `color{red}(ns, np, nd)` orbitals for hybridisation to yield a set of equivalent orbitals of definite geometry such as octahedral, tetrahedral, square planar and so on (Table 9.2).
`=>` These hybridised orbitals are allowed to overlap with ligand orbitals that can donate electron pairs for bonding. This is illustrated by the following examples.
`=>` It is usually possible to predict the geometry of a complex from the knowledge of its magnetic behaviour on the basis of the valence bond theory.
`color{red}("Example ")` : (i) In the diamagnetic octahedral complex, `color{red}([Co(NH_3)_6]^(3+))`, the cobalt ion is in `+3` oxidation state and has the electronic configuration `3d^6`. The hybridisation scheme is as shown in diagram.
● Six pairs of electrons, one from each `color{red}(NH_3)` molecule, occupy the six hybrid orbitals. Thus, the complex has octahedral geometry and is diamagnetic because of the absence of unpaired electron.
● In the formation of this complex, since the inner `color{red}(d)`-orbital (`color{red}(3d)`) is used in hybridisation, the complex, `color{red}([Co(NH_3)_6]^(3+))` is called an `text(inner orbital)` or `color{green}(("low spin")` or `color{green}("spin paired complex")`.
(ii) The paramagnetic octahedral complex, `color{red}([CoF_6]^(3–))` uses outer orbital (`color{red}(4d)`) in hybridisation `color{red}(sp^3 d^2)`.
● It is thus called `color{green}("outer orbital")` or `color{green}("high spin")` or `color{green}("spin free complex")`.
(iii) In tetrahedral complexes one `color{red}(s)` and three `color{red}(p)` orbitals are hybridised to form four equivalent orbitals oriented tetrahedrally.
● This is illustrated below for `color{red}([NiCl_4]^(2-))`. Here nickel is in `+2` oxidation state and the ion has the electronic configuration `3d^8`.
● The hybridisation scheme is as shown in diagram.
● Each `color{red}(Cl^-)` ion donates a pair of electrons.
● The compound is paramagnetic since it contains two unpaired electrons.
● Similarly, `color{red}([Ni(CO)_4])` has tetrahedral geometry but is diamagnetic since nickel is in zero oxidation state and contains no unpaired electron.
(iv) In the square planar complexes, the hybridisation involved is `color{red}(dsp^2)`.
● Example : `color{red}([Ni(CN)_4]^(2–))`.
● Here nickel is in `+2` oxidation state and has the electronic configuration `color{red}(3d^8)`.
● The hybridisation scheme is as shown in diagram.
● Each of the hybridised orbitals receives a pair of electrons from a cyanide ion.
● The compound is diamagnetic as evident from the absence of unpaired electron.
`color{red}("Note ")` : It is important to note that the hybrid orbitals do not actually exist. In fact, hybridisation is a mathematical manipulation of wave equation for the atomic orbitals involved.
`=>` These hybridised orbitals are allowed to overlap with ligand orbitals that can donate electron pairs for bonding. This is illustrated by the following examples.
`=>` It is usually possible to predict the geometry of a complex from the knowledge of its magnetic behaviour on the basis of the valence bond theory.
`color{red}("Example ")` : (i) In the diamagnetic octahedral complex, `color{red}([Co(NH_3)_6]^(3+))`, the cobalt ion is in `+3` oxidation state and has the electronic configuration `3d^6`. The hybridisation scheme is as shown in diagram.
● Six pairs of electrons, one from each `color{red}(NH_3)` molecule, occupy the six hybrid orbitals. Thus, the complex has octahedral geometry and is diamagnetic because of the absence of unpaired electron.
● In the formation of this complex, since the inner `color{red}(d)`-orbital (`color{red}(3d)`) is used in hybridisation, the complex, `color{red}([Co(NH_3)_6]^(3+))` is called an `text(inner orbital)` or `color{green}(("low spin")` or `color{green}("spin paired complex")`.
(ii) The paramagnetic octahedral complex, `color{red}([CoF_6]^(3–))` uses outer orbital (`color{red}(4d)`) in hybridisation `color{red}(sp^3 d^2)`.
● It is thus called `color{green}("outer orbital")` or `color{green}("high spin")` or `color{green}("spin free complex")`.
(iii) In tetrahedral complexes one `color{red}(s)` and three `color{red}(p)` orbitals are hybridised to form four equivalent orbitals oriented tetrahedrally.
● This is illustrated below for `color{red}([NiCl_4]^(2-))`. Here nickel is in `+2` oxidation state and the ion has the electronic configuration `3d^8`.
● The hybridisation scheme is as shown in diagram.
● Each `color{red}(Cl^-)` ion donates a pair of electrons.
● The compound is paramagnetic since it contains two unpaired electrons.
● Similarly, `color{red}([Ni(CO)_4])` has tetrahedral geometry but is diamagnetic since nickel is in zero oxidation state and contains no unpaired electron.
(iv) In the square planar complexes, the hybridisation involved is `color{red}(dsp^2)`.
● Example : `color{red}([Ni(CN)_4]^(2–))`.
● Here nickel is in `+2` oxidation state and has the electronic configuration `color{red}(3d^8)`.
● The hybridisation scheme is as shown in diagram.
● Each of the hybridised orbitals receives a pair of electrons from a cyanide ion.
● The compound is diamagnetic as evident from the absence of unpaired electron.
`color{red}("Note ")` : It is important to note that the hybrid orbitals do not actually exist. In fact, hybridisation is a mathematical manipulation of wave equation for the atomic orbitals involved.

